Cell and Gene Therapy Manufacturing Market to Surpass $23.4 Billion by 2033, Expanding at 15.2% CAGR| DataM Intelligence
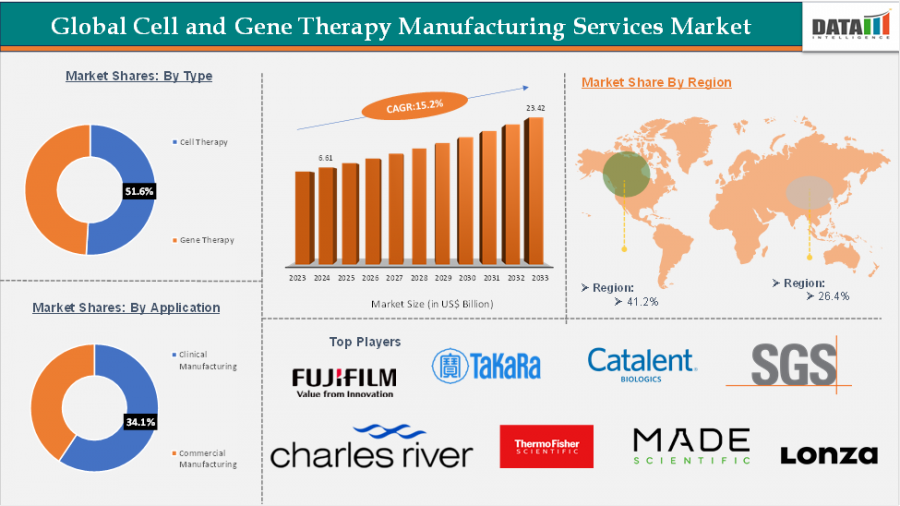
The Cell and Gene Therapy Manufacturing Services Market will reach $23.42 Bn by 2033, fueled by biotech growth, tech upgrades, and outsourcing trends.
NEW YORK, NY, UNITED STATES, June 17, 2025 /EINPresswire.com/ -- Market Overview :
The Cell and Gene Therapy Manufacturing Services Market has emerged as a cornerstone of the biopharmaceutical industry, with a market size of US$ 6.61 billion in 2024, projected to reach US$ 23.42 billion by 2033, growing at an impressive CAGR of 15.2% during 2025 and 2033. The rising number of clinical trials, regulatory approvals, and investments in regenerative medicine is accelerating demand for contract development and manufacturing organizations (CDMOs) that specialize in producing advanced therapy medicinal products (ATMPs).
Cell and gene therapies are reshaping the treatment landscape for cancer, genetic disorders, and autoimmune diseases. However, their manufacturing processes require highly specialized infrastructure, regulatory compliance, and scientific expertise making outsourcing an essential strategy for biotechnology companies.
Get Latest Sample Report Pdf : https://www.datamintelligence.com/download-sample/cell-and-gene-therapy-manufacturing-services-market
Market Drivers are :
Increasing clinical pipeline of cell and gene therapies fueling the need for scalable, GMP-compliant manufacturing support.
The high costs and operational complexity of in-house manufacturing are driving biotech companies to outsource to specialized CDMOs with proven expertise.
Growing regulatory support and fast-track designations for ATMPs in regions like the U.S., EU, and Japan.
Technological advancements in automation, closed-system bioprocessing, and viral vector platforms.
Rising investment from private equity and pharmaceutical giants in CGT-focused manufacturing capabilities.
Global shortage of viral vectors driving demand for specialized CDMO services.
Emergence of allogeneic therapies that require different manufacturing models and scale.
Key Players in the Market :
The market is driven by established and specialized CDMOs with deep expertise in handling the complexities of cell and gene therapy production. Leading companies include:
Charles River Laboratories
Thermo Fisher Scientific Inc.
Lonza
Merck KGaA
Catalent, Inc.
Takara Bio Inc.
FUJIFILM Irvine Scientific
Oxford Biomedica plc
SGS SA
Made Scientific
These firms offer services spanning plasmid development, viral vector manufacturing, cell line engineering, fill-finish, quality control, and regulatory support.
Market Segmentation :
By Type:
Cell Therapy Manufacturing
Gene Therapy Manufacturing
By Workflow:
Upstream Processing
Downstream Processing
Fill-Finish Operations
Analytical Testing & Quality Control
By Scale:
Preclinical
Clinical
Commercial
By Indication:
Oncology
Genetic Disorders
Neurological Disorders
Cardiovascular Diseases
Others
By Region:
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Latest News of USA -
In the U.S., 2024 was marked by heightened government backing for domestic cell and gene therapy manufacturing. The FDA launched a new CGT Advisory Program to streamline regulatory feedback, while companies like Catalent and Thermo Fisher expanded their U.S.-based viral vector facilities. Charles River Laboratories also opened a new development suite in Massachusetts aimed at supporting small biotech firms working on rare diseases.
Latest News of Japan -
Japan continues to lead in Asia-Pacific’s CGT development landscape. In 2024, Takara Bio announced the expansion of its manufacturing plant in Shiga to meet increasing demand for clinical-stage therapies. The Japanese government also pledged funding through its AMED (Japan Agency for Medical Research and Development) to strengthen local production capacity for regenerative medicines. FUJIFILM Irvine Scientific partnered with Japanese startups to supply custom media solutions for T-cell therapy development.
Recent Key Developments are :
Lonza launched an integrated CAR-T cell therapy manufacturing platform with faster batch turnaround and better yield.
Thermo Fisher Scientific acquired a viral vector development firm to strengthen its gene therapy portfolio.
Catalent finalized the expansion of its Maryland cell therapy facility, boosting production capacity for autologous treatment programs.
Oxford Biomedica signed a strategic collaboration with a European pharma company to produce lentiviral vectors at scale.
Merck KGaA invested in AI-powered quality control systems to speed up product release and minimize batch-related failures.
SGS SA introduced a new analytical service line to address regulatory expectations for potency assays.
Takara Bio advanced its automation systems to improve the efficiency and scalability of upstream AAV vector bioprocessing.
Charles River Laboratories launched a cell banking platform to serve clients from early development to commercial stages.
FUJIFILM Irvine Scientific developed a novel cell culture medium optimized for higher T-cell expansion in immunotherapies.
Made Scientific entered the U.S. market through a partnership with an oncology-focused biotech company.
Conclusion
The Cell and Gene Therapy Manufacturing Services Market stands at the frontier of innovation in modern medicine. As the demand for personalized, curative therapies continues to rise, the industry’s focus is shifting toward scalable, high-quality manufacturing solutions. Strategic partnerships, technological integration, and government incentives are shaping the next generation of CGT production. With biotech innovation on the rise and regulatory frameworks evolving in favor of fast-tracked therapies, CDMOs and service providers will play a pivotal role in bringing advanced treatments to market efficiently and safely.
Related Reports :
Sai Kumar
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release