Extracellular Vesicles from Peritoneal Adipose Stem Cells Promote Ovarian Cancer Progression via EGFR-NF-kB Activation

(A–C) The volcano plot showing differentially expressed genes between primary and malignant ascites OC tissues (A), malignant ascites and peritoneal OC tissues (B), and primary and peritoneal OC tissues (C). (D) The Venn diagram showing differentially exp
New Study Highlights Role of Adipose-Derived Stem Cells (ADSCs) in Ovarian Cancer Progression and Metastasis
CHINA, March 3, 2025 /EINPresswire.com/ -- Ovarian cancer (OC), the most lethal gynecological malignancy, is the fifth leading cause of cancer-related death in women. Despite advances in treatment, the primary challenges in overcoming ovarian cancer are relapse and metastasis. Hence, it is crucial to mechanistically investigate relapse and metastasis and foster novel therapeutic strategies.
This research, published in the Genes & Diseases journal by a team from the Tongji University School of Medicine, Naval Military Medical University, Shanghai University School of Medicine, and Shanghai Jiaotong University School of Medicine, focuses on peritoneal adipose-derived stem cells (ADSCs), a key component of the tumor microenvironment, as significant contributors to OC metastasis.
The researchers isolated adipocytes and ADSCs from OC patients and found that ADSCs enhanced OC proliferation and migration more than adipocytes. Notably, epidermal growth factor (EGF), a growth factor secreted by ADSCs, exhibited a significantly stronger effect on the proliferation and migration of OC cells than leptin, a major cytokine secreted by adipocytes.
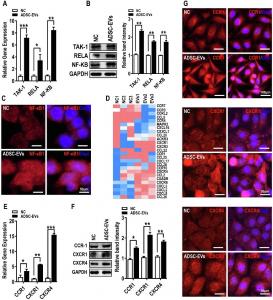
Additionally, transcriptome analysis unveiled the functional importance of extracellular vesicles (EVs) in mediating long-range signaling between peritoneal ADSCs and OC cells. The researchers found that ADSCs-EVs carried critical signaling molecules, such as EGF and epidermal growth factor receptor (EGFR), to OC cells, and upon fusion, these EVs activated key tumorigenic pathways, including the EGFR-NF-κB signaling axis, which is known to regulate inflammation, immune responses, and cancer progression.
Interestingly, the study further showed that blocking ADSCs-EVs production with a small molecule inhibitor, GW4869, or knocking down EGFR expression with shRNAs, effectively prevented the proliferation and migration of OC cells induced by ADSCs-EVs. These findings suggest that targeting the EV-mediated communication between ADSCs and OC cells may present a promising therapeutic approach for curbing OC metastasis.
Given the complex nature and the high mortality rate associated with advanced OC, further in vivo investigations are warranted to determine the role of peritoneal ADSCs-EVs in OC progression, metastasis, and drug resistance. In conclusion, the researchers highlight that these findings could open new avenues for the development of more effective treatments for OC by targeting the stromal cells within the peritoneal microenvironment.
Title of the original paper:
Peritoneal adipose stem cell-derived extracellular vesicles mediate the regulation of ovarian cancer cell proliferation and migration through EGFR-NF-κB signaling
Journal: Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI: https://doi.org/10.1016/j.gendis.2024.101283
Funding Information:
National Key R&D Program of China (No. 2019YFA0110300)
National Natural Science Foundation of China (No. 31900522, 81773302, 32370895, 82373269, 32070862)
Fundamental Research Funds for the Central Universities of China (project plan 22120210588)
Climbing Talent Program of Shanghai Tenth People's Hospital (China) (No. 2021SYPDRC036)
China Postdoctoral Science Foundation (No. 2019M651571)
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release